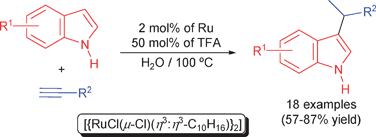
Ruthenium/TFA-catalyzed regioselective C-3-alkylation of indoles with terminal alkynes in water: efficient and unprecedented access to 3-(1-methylalkyl)-1H-indoles†
Abstract
An unprecedented C-3-alkylation reaction of indoles with terminal alkynes in aqueous medium has been developed using

 Please wait while we load your content...
Please wait while we load your content...