Coordination of NO2− ligand to Cu(i) ion in an O,O-bidentate fashion that evolves NO gas upon protonation: a model reaction relevant to the denitrification process†
Abstract
[(2,6-(Ph2P(o-C6H4)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2C5H3N)2Cu2](BF4)2 (2) has been prepared by treating 2,6-(Ph2P(o-C6H4)CH
N)2C5H3N)2Cu2](BF4)2 (2) has been prepared by treating 2,6-(Ph2P(o-C6H4)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2C5H3N (1) with [Cu(NCMe)4]BF4. Reaction of 2 and [Ph3PNPPh3]NO2 produces (2,6-(Ph2P(o-C6H4)CH
N)2C5H3N (1) with [Cu(NCMe)4]BF4. Reaction of 2 and [Ph3PNPPh3]NO2 produces (2,6-(Ph2P(o-C6H4)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2C5H3N)Cu(NO2) (3), with the
N)2C5H3N)Cu(NO2) (3), with the
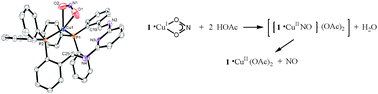

 Please wait while we load your content...
Please wait while we load your content...