Metal-catalyzed rearrangement of enantiomerically pure alkylidenecyclopropane derivatives as a new access to cyclobutenes possessing quaternary stereocenters†‡
Abstract
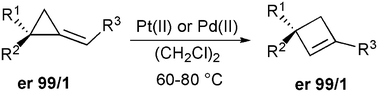
Pd(II)- and Pt(II)-catalyzed ring-expansion of enantiomerically pure alkylidenecyclopropane derivatives leads to the formation of cyclobutene species with a complete preservation of the stereogenic center.
- This article is part of the themed collection: Selective Catalysis for Organic Synthesis

 Please wait while we load your content...
Please wait while we load your content...