Tetrathiafulvalene (TTF) derivatives: key building-blocks for switchable processes
Abstract
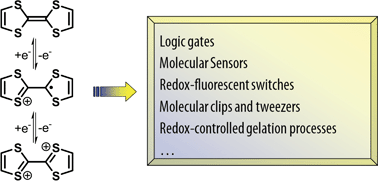
Besides a traditional use for the development of organic conducting materials, the tetrathiafulvalene (TTF) unit and its derivatives have recently appeared as key constituents for new applications, exploiting remarkable redox properties: a high π-donating ability and occurrence of three stable redox states. Indeed, in very recent years, an impressive variety of switchable TTF-based molecular and supramolecular (multifunctional) architectures have been designed and synthesized. In this feature article, we discuss recent developments of TTF-based molecular and supramolecular systems in this respect, including molecular sensors, redox-fluorescent switches, multi-input systems for logic gates, electrochemically-driven conformational controls, molecular clips and tweezers, and redox-controlled gelation processes.
- This article is part of the themed collection: ChemComm 60th Anniversary Historic Papers from China

 Please wait while we load your content...
Please wait while we load your content...