Deprotonative cadmation of functionalized aromatics†
Abstract
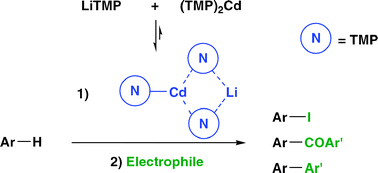
This communication describes the deproto-metalation of a large range of aromatics including heterocycles using a newly developed lithium–cadmium base; the reaction proceeds at room temperature with an excellent chemoselectivity and efficiency, and proved to be regioselective in most cases.

 Please wait while we load your content...
Please wait while we load your content...