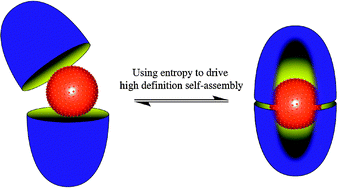
High definition self-assemblies, those that possess order at the molecular level, are most commonly made from subunits possessing metals and metal coordination sites, or groups capable of partaking in hydrogen bonding. In other words, enthalpy is the driving force behind the free energy of assembly. The hydrophobic effect engenders the possibility of (nominally) relying not on enthalpy but entropy to drive assembly. Towards this idea, we describe how template molecules can trigger the dimerization of a cavitand in aqueous solution, and in doing so are encapsulated within the resulting capsule. Although not held together by (enthalpically) strong and directional non-covalent forces, these capsules possess considerable thermodynamic and kinetic stability. As a result, they display unusual and even unique properties. We discuss some of these, including the use of the capsule as a nanoscale reaction chamber and how they can bring about the separation of hydrocarbon gases.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...