19F NMR based pH probes: lanthanide(iii) complexes with pH-sensitive chemical shifts†
Abstract
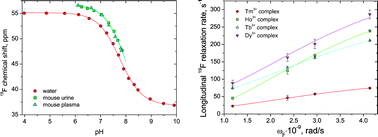
Experimental measurements and theoretical analysis of magnetic properties, structural dynamics and acid–base equilibria for several lanthanide(III) complexes with tetraazacyclododecane derivatives as

 Please wait while we load your content...
Please wait while we load your content...