Direct asymmetric catalytic 1,2-addition of RZnX to aldehydes promoted by AlMe3 and reversal of expected stereochemistry†
Abstract
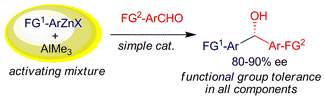
Addition of AlMe3 to commercial THF solutions of RZnX (R = aryl, functionalised aryl, vinyl; X = Br, I) simultaneously promotes Schlenk equilibria (leading to competent nucleophiles) and the formation of an Al–Zn-ligand catalyst delivering 80–90% ee for Ar1CH(OH)Ar2 formation from aldehydes.

 Please wait while we load your content...
Please wait while we load your content...