The asymmetric vinylogous Mannich reaction of dicyanoalkylidenes with α-amido sulfones under phase-transfer conditions†
Abstract
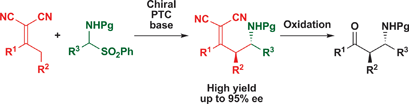
The stereoselective vinylogous Mannich reaction of dicyanoalkylidenes under phase-transfer catalytic conditions utilizing stable α-amido

 Please wait while we load your content...
Please wait while we load your content...