A versatile synthesis of diverse 3,4-fused cinnolines via the base-catalysed condensation of 2-amino-2′-nitrobiaryls†
Abstract
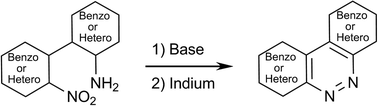
Benzo[c]cinnolines, thieno[3,2-c]cinnolines, pyrido[3,2-c]cinnolines and the previously undescribed quinoxalino[6,7-c]cinnoline ring system are conveniently prepared by a short synthetic route comprised of Suzuki coupling, base-catalysed cyclisation and deoxygenation. The use of tandem borylation–Suzuki coupling further extends the scope of this process to include highly substituted benzo[c]cinnolines.

 Please wait while we load your content...
Please wait while we load your content...