Supermolecule density functional calculations suggest a key role for solvent in alkaline hydrolysis of p-nitrophenyl phosphate†
Abstract
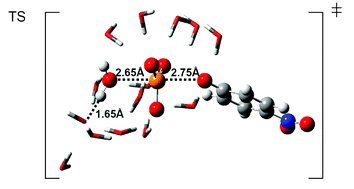
Supermolecule density functional theory calculations show that solvent is responsible for the concerted transition state in alkaline hydrolysis of p-nitrophenyl phosphate suggested by heavy atom kinetic isotope effects.

 Please wait while we load your content...
Please wait while we load your content...