Synthesis and structural characterisation as 12-helix of the hexamer of a β-amino acid tethered to a pyrrolidin-2-one ring†
Abstract
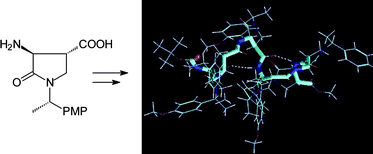
Starting from (3S,4R,1′S)-3-amino-2-oxo-1-[1′-(4-methoxyphenylethyl)]pyrrolidine carboxylic acid (2), the first synthesis of a β-foldamer containing pyrrolidin-2-one rings is described, whose 12-helix conformation is assigned by

 Please wait while we load your content...
Please wait while we load your content...