A modular approach to the synthesis of 2,3,4-trisubstituted tetrahydrofurans†
Abstract
A highly diastereoselective Lewis acid-mediated [1,3] rearrangement of 1,3-dioxepins is the key step along a modular route to 2,3,4-trisubstituted tetrahydrofurans.
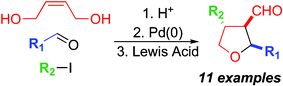

 Please wait while we load your content...
Please wait while we load your content...