Extended ethidium bromide analogue as a triple helix intercalator: synthesis, photophysical properties and nucleic acids binding†
Abstract
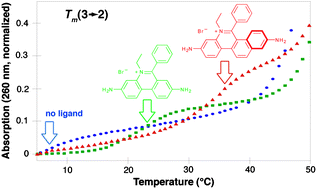
Ethidium bromide has been extended by fusing an additional aromatic ring resulting in a larger intercalator with increased affinity for poly r(A)·r(U), poly d(A)·d(T) and triple helices when compared to the parent heterocycle.

 Please wait while we load your content...
Please wait while we load your content...