Synthesis of chiral cyclic oligothiazolines: a novel structural motif for a macrocyclic molecule†
Abstract
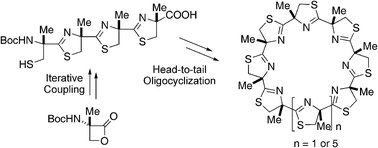
The synthesis of chiral cyclic oligo(4-β-methyl)thiazolines is described; linear oligothiazolines were efficiently prepared by the iterative formation of a thiazoline ring and a two-directional block condensation, and construction of 24- to 36-membered cyclic oligothiazoline systems could be achieved by the head-to-tail cyclooligomerization of doubly deprotected linear fragments and subsequent thiazoline formation.

 Please wait while we load your content...
Please wait while we load your content...