A direct organocatalytic entry to sphingoids: asymmetric synthesis of d-arabino- and l-ribo-phytosphingosine
Abstract
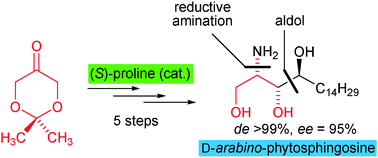
The organocatalytic asymmetric synthesis of D-arabino- and L-ribo-phytosphingosine is described employing a diastereo- and enantioselective (S)-proline-catalyzed

 Please wait while we load your content...
Please wait while we load your content...