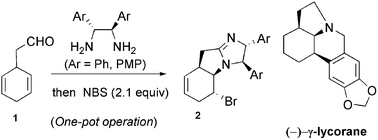
Intramolecular bromo-amination of 1,4-cyclohexadieneaminal: one-pot discrimination of two olefins and concise asymmetric synthesis of (−)-γ-lycorane†
Abstract
The reaction of cyclohexa-2,5-dienyl-1-methylaldehyde and optically pure 1,2-diaryl-1,2-diamine followed by intramolecular bromo-amination produced a one-pot discrimination of two

 Please wait while we load your content...
Please wait while we load your content...