Unprecedented asymmetric induction through configurationally stable lithium N-(α-methylbenzyl)phosphinamides. A new entry to enantiomerically pure γ-aminophosphinic acids and esters†‡
Abstract
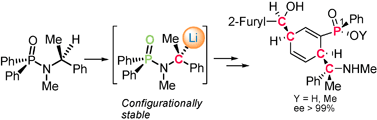
The first examples of configurationally stable N-benzyl-N-phosphinoyl carbanions are described. Their applications to the synthesis of homochiral γ-aminophosphinic acids and

 Please wait while we load your content...
Please wait while we load your content...