Total synthesis of buergerinin F via effective construction of the asymmetric quaternary carbons using an enantioselective aldol reaction†
Abstract
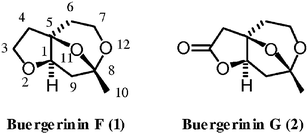
An efficient method for the synthesis of (+)-buergerinin F is established via the enantioselective aldol reaction of a tetrasubstituted

 Please wait while we load your content...
Please wait while we load your content...