Radical-carbanion cyclo-coupling in armed aromatics: overriding steric hindrance to ring closure
Abstract
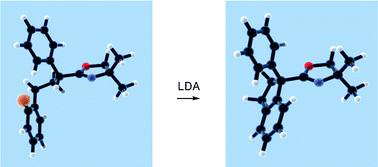
ω-(2-Halophenyl)alkyl-2-oxazolines were prepared and reacted via base promoted intramolecular coupling of radical with carbanionic centres to yield 1-phenyl-1-oxazolino-indan and -tetralin derivatives containing quaternary C-atoms.

 Please wait while we load your content...
Please wait while we load your content...