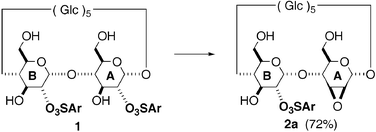
The first hetero-bifunctionalization of the secondary face of β-cyclodextrin: selective and efficient conversion of the A-ring of a 2A,2B-disulfonate to 2A,3A-epoxymannoside
Abstract
The A-ring of 2A,2B-O,O-di(mesitylenesulfonyl)-β-cyclodextrin was converted to 2A,3A-epoxymannoside without affecting the other sulfonylated residue, which affords the first approach to hetero-bifunctionalization at the secondary hydroxyl side of

 Please wait while we load your content...
Please wait while we load your content...