A modular design of ruthenium catalysts with diamine and BINOL-derived phosphinite ligands that are enantiomerically-matched for the effective asymmetric transfer hydrogenation of simple ketones†
Abstract
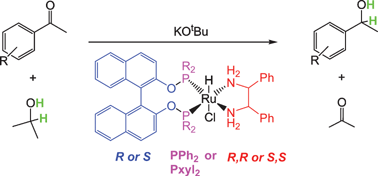
A method is reported for making a potentially very wide series of ruthenium hydrido chloro complexes with

 Please wait while we load your content...
Please wait while we load your content...