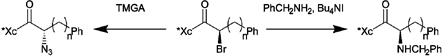
Controlling diastereoselectivity in the reactions of enantiomerically pure α-bromoacyl-imidazolidinones with nitrogen nucleophiles: substitution reactions with retention or inversion of configuration†‡
Abstract
Diastereoselective substitution reactions of α-bromoacyl-imidazolidinones with

 Please wait while we load your content...
Please wait while we load your content...