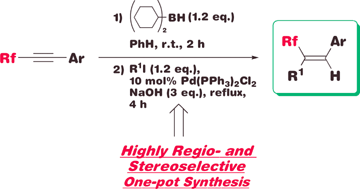
A sequential highly stereoselective hydroboration and Suzuki–Miyaura cross-coupling reaction of fluoroalkylated internal acetylenes: a practical one-pot synthesis of fluoroalkylated trisubstituted alkenes
Abstract
The one-pot synthesis of trisubstituted

 Please wait while we load your content...
Please wait while we load your content...