3-Chloropropenyl pivaloate in organic synthesis: the first asymmetric catalytic entry to syn-alk-1-ene-3,4-diols
Abstract
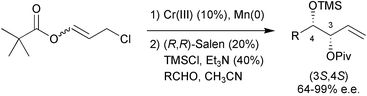
The first asymmetric catalytic synthesis of syn-alk-1-ene-3,4-diols was developed; the regio-, diastereo- and enantioselective addition of

 Please wait while we load your content...
Please wait while we load your content...