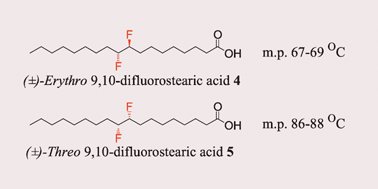
The fluorine gauche effect. Langmuir isotherms report the relative conformational stability of (±)-erythro- and (±)-threo-9,10-difluorostearic acids†
Abstract
(±)-Erythro- and (±)-threo- 9,10-difluorostearic acids, which differ only by a stereogenic interconversion of a single C–F bond, have significantly different conformational stabilities.

 Please wait while we load your content...
Please wait while we load your content...