Synthesis of γ-methylene oxacycles and α- and β-alkylidene lactonesvia silicon-assisted ring opening of cyclopropyl carbinols†
Abstract
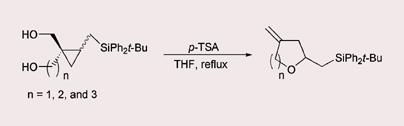
Cyclopropyl carbinols bearing a (tert-butyldiphenylsilyl)methyl substituent undergo silicon-assisted regioselective ring cleavage and the resulting β-silyl carbocation is intramolecularly trapped with

 Please wait while we load your content...
Please wait while we load your content...