Spatial organization of biochemical cues in 3D-printed scaffolds to guide osteochondral tissue engineering†
Abstract
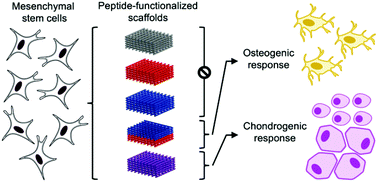
Functional repair of osteochondral (OC) tissue remains challenging because the transition from bone to cartilage presents gradients in biochemical and physical properties necessary for joint function. Osteochondral regeneration requires strategies that restore the spatial composition and organization found in the native tissue. Several biomaterial approaches have been developed to guide chondrogenic and osteogenic differentiation of human mesenchymal stem cells (hMSCs). These strategies can be combined with 3D printing, which has emerged as a useful tool to produce tunable, continuous scaffolds functionalized with bioactive cues. However, functionalization often includes one or more post-fabrication processing steps, which can lead to unwanted side effects and often produce biomaterials with homogeneously distributed chemistries. To address these challenges, surface functionalization can be achieved in a single step by solvent-cast 3D printing peptide-functionalized polymers. Peptide-poly(caprolactone) (PCL) conjugates were synthesized bearing hyaluronic acid (HA)-binding (HAbind–PCL) or mineralizing (E3–PCL) peptides, which have been shown to promote hMSC chondrogenesis or osteogenesis, respectively. This 3D printing strategy enables unprecedented control of surface peptide presentation and spatial organization within a continuous construct. Scaffolds presenting both cartilage-promoting and bone-promoting peptides had a synergistic effect that enhanced hMSC chondrogenic and osteogenic differentiation in the absence of differentiation factors compared to scaffolds without peptides or only one peptide. Furthermore, multi-peptide organization significantly influenced hMSC response. Scaffolds presenting HAbind and E3 peptides in discrete opposing zones promoted hMSC osteogenic behavior. In contrast, presenting both peptides homogeneously throughout the scaffolds drove hMSC differentiation towards a mixed population of articular and hypertrophic chondrocytes. These significant results indicated that hMSC behavior was driven by dual-peptide presentation and organization. The downstream potential of this platform is the ability to fabricate biomaterials with spatially controlled biochemical cues to guide functional tissue regeneration without the need for differentiation factors.
- This article is part of the themed collection: Biomaterials Science Emerging Investigators 2021

 Please wait while we load your content...
Please wait while we load your content...
