Substrate stiffness induced mechanotransduction regulates temporal evolution of human fetal neural progenitor cell phenotype, differentiation, and biomechanics†
Abstract
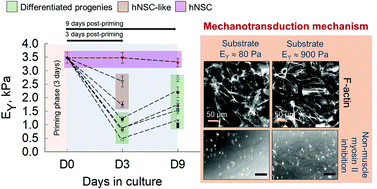
While the mechanotransduction-induced fate of adult neural stem/progenitor cells (NPCs) is relatively known, how substrate stiffness regulates the temporal evolution of the biomechanics and phenotype of developmentally relevant human fetal NPCs (hNPCs) and their mechanosensing pathways remain unknown. Here, we primed hNPCs on tissue-culture plastic (TCPS) for 3 days in non-differentiating medium before transferring to TCPS or Geltrex™ gels (<1 kPa) for 9-day cultures post-priming, and regularly assessed stemness, differentiation, and cell mechanics (Young's modulus, tether forces, apparent membrane tension, tether radius). hNPCs maintained stemness on TCPS while those on gels co-expressed stemness and neural/glial markers, 3-days post-priming. Biomechanical characteristics remained unchanged in cells on TCPS but were significantly altered in those on gels, 3-days post-priming. However, 9-days post-priming, hNPCs on gels differentiated, with significantly more neurons on softer gels and glia on stiffer gels, while those on TCPS maintained their native stemness. Withdrawal of bFGF and EGF in 9-day cultures induced hNPC differentiation and influenced cell mechanics. Cells on stiffer gels had higher biomechanical properties than those on softer gels throughout the culture period, with NPC-like > neural > glia subtypes. Higher stress fiber density in cells on stiffer gels explains their significantly different biomechanical properties on these gels. Blebbistatin treatment caused cell polarization, lowered elastic modulus, and enhanced tether forces, implicating the role of non-muscle myosin-II in hNPC mechanosensing, adaptability, and thereby mechanics. Such substrate-mediated temporal evolution of hNPCs guide design of smart scaffolds to investigate morphogenesis, disease modeling, stem cell biology, and biomaterials for tissue engineering.

 Please wait while we load your content...
Please wait while we load your content...
