Membrane deformation and layer-by-layer peeling of giant vesicles induced by the pore-forming toxin pneumolysin†
Abstract
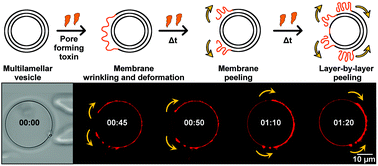
Protein–membrane interactions that modify the shape of membranes are important for generating curvature, membrane deformation by protein–protein crowding or trafficking of vesicles. Giant vesicles represent a simplified but versatile model for biological membranes and are commonly employed for the study of lipid domains and permeation across compartments. In this study, we investigated the interaction of pneumolysin (PLY), a pore-forming toxin secreted by Streptococcus pneumoniae, with multilamellar and unilamellar membranes. It reveals an enlargement of membrane area due to the insertion of pores into the bilayer and protein–membrane aggregations that induce membrane deformation and wrinkling. Moreover, we demonstrate that PLY peel-off layers from multilamellar giant vesicles in a hitherto unknown layer-by-layer peeling mechanism, which reveals the structure and number of membrane lamellae. We employed microfluidic methods to capture giant vesicles and confocal laser scanning microscopy, transmission microscopy, dynamic light scattering and cryo-electron microscopy to disclose the structure of multilamellar vesicles. Based on our findings we suggest how back-to-back pore arrangements stabilize large PLY–membrane entities and that pore-displaced lipids possibly remain in the membrane.


 Please wait while we load your content...
Please wait while we load your content...