Synthesis of controlled, high-molecular weight poly(l-glutamic acid) brush polymers†
Abstract
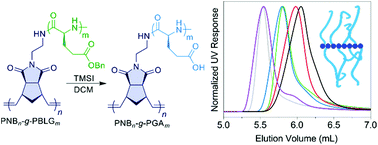
We report the synthesis and characterization of high-molecular weight poly(L-glutamic acid) based brush polymers. Utilizing a combination of ring-opening metathesis polymerization of norbornene based monomers and ring-opening polymerization of γ-benzyl-L-glutamate N-carboxyanhydride, high-molecular weight γ-benzyl protected poly(L-glutamic acid) brush polymers are synthesized. Controlled and complete deprotection of the benzyl groups using trimethylsilyl iodide resulted in poly(L-glutamic acid) based brush polymers with molecular weights up to 3.6 MDa, which may potentially be used to prepare size-controlled unimolecular polymeric nanomedicine for drug delivery applications. Camptothecin brush poly(L-glutamic acid) conjugates were prepared and their stability, drug release kinetics, and in vitro toxicity were studied.


 Please wait while we load your content...
Please wait while we load your content...