A molecular dynamics study on pH response of protein adsorbed on peptide-modified polyvinyl alcohol hydrogel†
Abstract
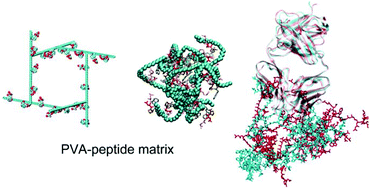
The interactions between proteins and functional biomaterials under different physical and environmental conditions need to be understood when designing biomedical devices. Herein, we present a molecular dynamics simulation study of the fragment antigen-binding (Fab) of trastuzumab (a monoclonal antibody) and its complex with a peptide-modified polyvinyl alcohol (PVA) hydrogel at different pH values. Consistent with experiments, PVA when modified by charged ligands does shrink as a direct response to a drop in the pH. The protein maintains a stable conformation when adsorbed on the hydrogel matrix with a varied pH, showing no signs of denaturation in all simulated systems, suggesting that peptide-grafted PVA is a good biocompatible material. Under neutral conditions, the hydrogel alone stabilizes the interactions between the protein and the peptide ligands. Strikingly under acidic conditions the protein–ligand interactions are disrupted by a collective protonation of ligands. A sharp decrease in the interaction energies, accompanied by the sudden increase of the protein–ligand distance, indicates a rapid pH response in the protein–hydrogel complex. This will be important in protein delivery and purification. The effect of pH on the interactions and the dynamics of the protein and the sudden pH response of the hydrogel at the atomic level present a new functional perspective in developing new hydrogels with desirable properties.

 Please wait while we load your content...
Please wait while we load your content...