A dual-mode colorimetric sensor based on copper nanoparticles for the detection of mercury-(ii) ions†
Abstract
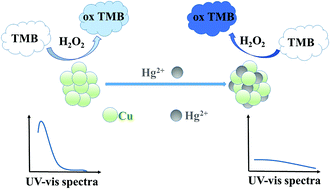
In this study, water-soluble citrate-capped copper nanoparticles (Cu NPs) were synthesized by a simple and rapid method. We found that these citrate-capped Cu NPs possessed an intrinsic peroxidase-like activity, which could catalyse the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) to generate a light blue product in the presence of hydrogen peroxide (H2O2). It was also found that mercury ions (Hg2+) could enhance the peroxidase-like activity of the citrate-capped Cu NPs, causing the colour to turn bright blue. The colour change was dependent on the concentration of Hg2+. Therefore, a colorimetric method for Hg2+ detection was established with a linear range from 0.050 μM to 10.000 μM and a detection limit of 0.185 Mm. More interestingly, citrate-capped Cu NPs have a characteristic absorption peak at 260 nm, we also found that Hg2+ could cause the absorption peak at 260 nm to change. Therefore, we developed another colorimetric method for Hg2+ detection based on the absorption peak of citrate-capped Cu NPs at 260 nm. This colorimetric method was shown to enable convenient and sensitive quantification of Hg2+ in the concentration range of 0.100 μM to 6.000 μM with a limit of detection of 0.052 μM. In this study, a dual-mode sensor for detection of Hg2+ was constructed, which exhibited good sensitivity and selectivity.


 Please wait while we load your content...
Please wait while we load your content...