Determination of phosphate anions with a near-infrared heptamethine cyanine dye in a neutral aqueous solution
Abstract
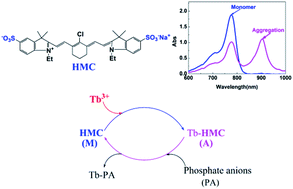
A novel absorbance recovery method has been developed for the determination of phosphate anions with a near-infrared reagent. This method employs a two-reagent system composed of anionic heptamethine cyanine (HMC) and Tb3+. The absorbance of HMC, with a maximum peak at 778 nm, was decreased due to addition of Tb3+ in appropriate concentrations, but recovered when phosphate anions were added. Under optimal conditions, the reciprocal extent of recovered absorbance was proportional to the concentration of phosphate anions. The calibration curves are linear over the range of 4.00 × 10−7 to 8.00 × 10−6 M for H2PO4−; 1.00 × 10−7 to 7.00 × 10−6 M for P2O74−; 5.00 × 10−8 to 1.00 × 10−6 M for adenosine diphosphate (ADP) and 5.00 × 10−8 to 8.00 × 10−7 M for adenosine triphosphate (ATP). Because of the specific affinity of phosphate anions for Tb3+, there is minimal interference from other ions. This method has been successfully applied to the determination of inorganic phosphate anions in human saliva samples with satisfactory results.


 Please wait while we load your content...
Please wait while we load your content...