Development of a robust SFC method for evaluation of compatibility for a novel antituberculotic fixed-dose combination†
Abstract
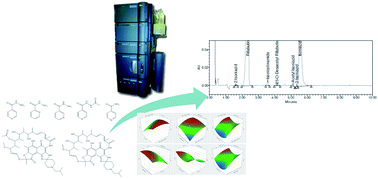
A fast and simple supercritical fluid chromatography method for the simultaneous determination of two antituberculotic drugs, isoniazid and rifabutin, and their impurities from a new proposed fixed-dose combination (FDC) was developed. Isoniazid and rifabutin are used in antituberculotic therapy as single active substance medications. Although FDCs have proved beneficial in general, compatibility issues for some combinations have been reported. The development of a suitable analytical method was, therefore, necessary to assess the applicability of these two active substances for a new FDC formulation. Two active components and their related compounds were successfully separated in only 7 min using an Acquity UPC2™ Torus DEA, 130 Å, 1.7 μm, 3.0 × 100 mm column. Compressed CO2 was modified with a mixture of methanol–2-propanol–water–triethylamine, in ratio 70 : 28 : 2 : 0.1 (v/v/v/v), to obtain gradient elution. Quality-by-design principles were implemented to accomplish a reliable and robust analytical method. Modeling software (JMP®, version 13.2.1) was utilized for the Design of Experiments. Mathematical models for the relationship of column temperature, pressure, modifier composition and flow rate to five critical quality attributes were generated. A comprehensive method development approach facilitated detection of influencing factors and significant second-order terms indicating factors' interaction and nonlinear effects. The new proposed method was successfully validated for specificity, linearity, sensitivity, accuracy, precision, stability, filter study and robustness in compliance with internationally accepted guidelines. Acceptable validation results were obtained for both concentration level ranges (assay and impurities determination). Finally, the applicability of the method was tested for commercially available samples of both active substances.


 Please wait while we load your content...
Please wait while we load your content...