Single-labeled peptide substrates for detection of protease activity based on the inherent fluorescence quenching ability of Cu2+
Abstract
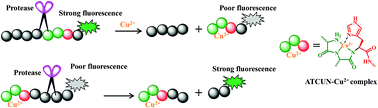
A general peptide substrate for the specific detection of proteases is linked with a quencher/fluorophore pair. However, labeling of the substrate with a quencher/fluorophore pair may increase the complexity for the synthesis of peptides and affect the approach of protease to the cleavage site. A peptide with a specific sequence has been shown to bind Cu2+ with high affinity. More intriguingly, Cu2+ exhibits a high inherent quenching ability towards the fluorophore by complexation with the sequence-specific peptide. Based on this fact, this work reports a general single-labeled peptide substrate for the detection of protease activity, screening of potential inhibitors and evaluation of cell apoptosis. The feasibility and applications of the method were demonstrated by assays of caspase-3 and β-secretase with a signal-on and a signal-off format, respectively.


 Please wait while we load your content...
Please wait while we load your content...