Analytical Quality by Design (AQbD) as a multiaddressable platform for co-encapsulating drug assays†
Abstract
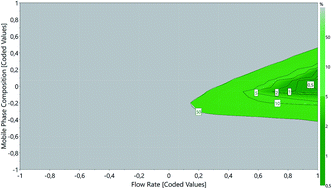
The current study describes the application of an Analytical Quality by Design (AQbD) approach for the selection and optimization of a reversed-phase high performance liquid chromatography (RP-HPLC) method to simultaneously quantify curcumin, atorvastatin calcium and celecoxib in lipid nanoparticles. Risk assessment was carried out to set the risk factors that may have a significant influence on method performance. Mobile phase composition and flow rate were identified as critical method parameters, and a 3k full factorial design with center point was applied to understand their impact on the number of theoretical plates, retention time, peak resolution, tailing factor and sensitivity. Statistical modeling, followed by response surface analysis led to the characterization of the method operable design region (MODR) and the selection of the most suitable chromatographic conditions for validation, following Food and Drug Administration and International Council on Harmonisation recommendations. The mobile phase was composed of 2% v/v glacial acetic acid : acetonitrile (50 : 50, v/v) and was eluted at an isocratic flow rate of 1.0 mL min−1. The analyses were performed on a Kinetex® EVO C18 column (Torrance, USA), with 5 μm particle size, 4.6 μm internal diameter and 150 mm length, at 35 °C and under UV-vis detection at 425 (curcumin), 247 (atorvastatin calcium) and 250 nm (celecoxib). Retention time values of curcumin, atorvastatin calcium and celecoxib were found to be 4.520, 5.033 and 7.576 min, respectively. AQbD provided a comprehensive framework for developing a reliable, effective, flexible band robust method for the routine analysis of the compounds in quality control laboratories.


 Please wait while we load your content...
Please wait while we load your content...