A novel near-infrared and naked-eyes turn on fluorescent probe for detection of biothiols with a large Stokes shift and its application in living cells†
Abstract
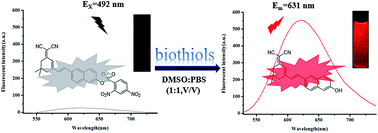
A novel turn-on fluorescent probe (DDND) for highly selective detection of biothiols over other amino acids was synthesized and investigated in this work, which used the 2,4-dinitrobenzenesulfonyl (DNBS) group as a fluorescent quencher. The novel fluorophore (HDM) features a large π-conjugation system and a typical intramolecular charge transfer (ICT) process and has a long emission wavelength at 623 nm as well as a large Stokes shift (λem − λex = 131 nm). Besides that, this red-emitting probe exhibited good linearity ranges with a low detection limit of 0.23 μM for Cys, 0.34 μM for Hcy and 0.41 μM for GSH respectively. Upon titration of thiols, the color of the solution changed from yellow to dark red, which means it can be detected by naked eyes. Finally, probe DDND was successfully applied to bioimage intracellular Cys in HeLa cells with low cytotoxicity.


 Please wait while we load your content...
Please wait while we load your content...