A novel way for the determination of the acid values of edible oils using a phthalocyanine derivative†
Abstract
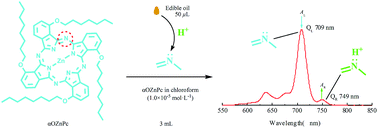
To effectively determine acid values (AVs) of edible oils by UV-vis spectroscopy, a novel method is developed based on the two characteristic peaks of tetra-α-octyloxy zinc phthalocyanine at 709 and 749 nm. They are sensitive to trace fatty acid in chloroform, and especially the one at 749 nm shows a good correlation between absorbance (AR) and fatty acid molar concentration (cb). The functional relationship can be expressed as AR = 0.08052 ln cb + 1.01100 suitable for common edible oils with AVs 0.06–2.40 mg KOH g−1. The analysis only needs 50 μL, about a drop, of edible oil mixed in 3 mL solution of the phthalocyanine compound in chloroform (1.0 × 10−5 mol L−1) to record AR and then calculate cb from which AV is determined. For the edible oils with larger AVs exceeding 2.40 mg KOH g−1, they can be diluted and analyzed by the UV-vis method. For those with minimal AVs no more than 0.06 mg KOH g−1, the AVs can be obtained by another supplementary equation AR = 3429.3cb + 0.073951, applicable to the range of 0.0024–0.06 mg KOH g−1. The UV-vis method is not only more convenient for the determination of AVs of edible oils but also hardly disturbed by their color in general, and thus it has great potential for application in the analysis of oil products.


 Please wait while we load your content...
Please wait while we load your content...