Piperazine-tuned NBD-based colorimetric and fluorescent turn-off probes for hydrogen sulfide†
Abstract
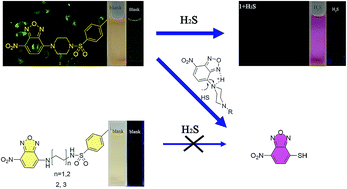
The piperazine ring is a very important factor in governing molecular structure to generate the special properties of a compound. New NBD-based fluorescent probes 1–3 were designed and synthesized for hydrogen sulfide (H2S) detection. Probe 1 showed high sensitivity and selectivity for H2S with a color change from yellow to pink for naked eye observation. However, the analogues (2 and 3) were response-negative toward H2S. In order to clarify the importance of the piperazine ring in the NBD-based H2S probe and the mechanism, the crystal structure of 1 was obtained and the piperazine ring was deemed to mediate H2S nucleophilic addition. In addition, probe 1 had limits of detection of 18.96 μM (UV) and 23.42 μM (FL), and the thiolysis rate for 1 was found to be 0.49 M−1 s−1. Living cell imaging results indicated that probe 1 could be utilized to trace intracellular H2S. This work has a great guiding role in the design of NBD-based fluorescent probes through a nucleophilic addition mechanism.


 Please wait while we load your content...
Please wait while we load your content...