A nitrogen doped carbon quantum dot-enhanced chemiluminescence method for the determination of Mn2+
Abstract
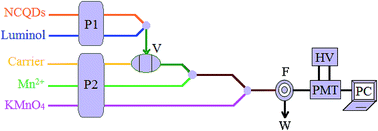
Nitrogen doped carbon quantum dots (NCQDs) were synthesized via an ultrasonic method with ascorbic acid as the carbon source and ammonia as the nitrogen source. The synthesized NCQDs could significantly enhance the chemiluminescence (CL) reaction between luminol and KMnO4 under alkaline conditions based on its catalysis. The increased CL intensity also depended on the synthetic temperature and time of NCQDs. Trace amounts of Mn2+ could cause a visible restraining of the CL intensity of the NCQD–KMnO4–luminol system because of the formation of complexes between Mn2+ and some groups such as NH– and CH2– on the surface of NCQDs, which resulted in the aggregation of NCQDs and scavenging of O2˙− free radicals and then the catalytic capacity reduction of NCQDs. Under optimum conditions, the decrease in CL intensity was proportional to the concentration of Mn2+ in the range of 0.3 to 50.0 µM. The detection limit (3σ) was 43.0 nM. The feasibility of the method was also demonstrated for determining Mn2+ concentration in water samples.


 Please wait while we load your content...
Please wait while we load your content...