An on-flow assay for screening of β-secretase ligands by immobilised capillary reactor-mass spectrometry
Abstract
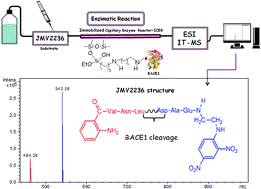
The enzyme β-secretase1 (BACE1) is an aspartyl protease that initiates the cleavage of the Aβ amyloid precursor protein (APP), to generate and aggregate β-amyloid (Aβ) peptides. Aggregates of Aβ peptides are implicated in the pathogenesis of Alzheimer's disease (AD), so inhibition of BACE1 is an attractive therapeutic strategy to treat AD. Many methods involving the principle of fluorescence resonance energy transfer (FRET) have been developed to evaluate the inhibitory activity of different compounds against enzymes. Because these methods have several drawbacks, studies involving mass spectrometry (MS) detection have been carried out. In this study, we prepared a BACE1 immobilised capillary enzyme reactor (ICER) attached to a mass spectrometer for the on-flow screening of ligands. First, we immobilised BACE1 on a fused silica capillary (BACE1-ICER, 30 cm × 0.1 mm ID) using glutaraldehyde as a spacer. We then used JMV2236, a peptide that mimics the Swedish mutated APP (amyloid protein precursor) sequence, as the substrate to characterise BACE1-ICER. Finally, we validated the method and investigated the kinetic parameter (KMap). We established the best conditions to accomplish coupling and inhibition studies by liquid chromatography-ion trap-mass spectrometry (LC-IT-MS) (inhibitory potency and mechanism of action) of the standard β-secretase inhibitor. The BACE1-ICER-MS method was reproducible and stable. The parameter KMap was 17.2 ± 5 μmol L−1, which demonstrated that immobilisation did not significantly change the structure, flexibility, or conformation of BACE1 or even the accessibility to the binding sites in the enzyme. IC50 and Ki were 1.19 ± 0.2 μmol L−1 and 0.96 ± 0.01 μmol L−1 for the standard β-secretase inhibitor, respectively, which confirmed that BACE1-ICER identifies the reference inhibitor at the micromole scale. BACE1-ICER remained active and stable along the development of the method and of the studies conducted herein and maintained 43% of residual activity after being used for ten months.


 Please wait while we load your content...
Please wait while we load your content...