Colorimetric probe for copper(ii) ion detection based on cost-effective aminoquinoline derivative
Abstract
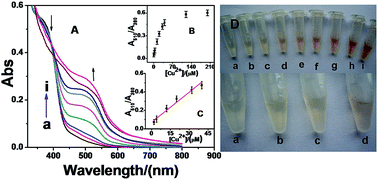
A readily available aminoquinoline derivative (5-(quinolin-8-yliminol)pentanal, QYP) is used as a new cost-effective colorimetric probe for Cu2+ ion detection by forming a complex with a stoichiometry of 1 : 2. The QYP conjugated with Cu2+ ions induces a discernable color change from light yellow to red-brown in the solution, and the corresponding UV-Vis absorption shifts from 380 nm to 510 nm dramatically. The detection limit for Cu2+ ions is 2.0 μM by the naked eye, approaching other previously reported colorimetric methods, and without tedious conditions. Other possible contaminated metal ions cause no significant interference to detecting Cu2+ ions with the proposed probe. The stability constant of the complex is estimated to be 7.87 × 1010 M−2, which suggests the high stability of the conjugation of QYP with Cu2+ ions. Besides, it was successfully applied to assay Cu2+ ions in river water samples, indicating that the probe may become a great potential candidate in complex sample determination.


 Please wait while we load your content...
Please wait while we load your content...