Determination of mercury(ii) ions in aqueous solution using silver nanorods as a probe†
Abstract
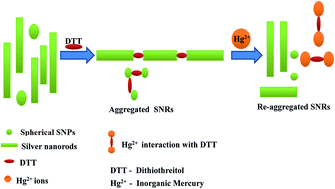
We report here the selective and sensitive determination of Hg2+ in aqueous solution using the aggregation and re-aggregation of silver nanorods (SNRs) in the presence of dithiothreitol (DTT). The SNRs covalently bound with the thiol groups at the ends of the DTT molecule, leading to the aggregation of the SNRs. However, the aggregation of the SNRs was prevented by the addition of Hg2+. The thiol group of DTT has a strong binding affinity with Hg2+ that helps to prevent the aggregation of SNRs. UV-visible spectrophotometry and transmission electron microscopy confirmed the aggregation and re-aggregation of SNRs with DTT and Hg2+. The absorption ratio (A/A0) of the SNRs at λ585 nm decreased after reaction with DTT and increased with the addition of Hg2+. The changes in the absorption ratio of the SNRs at λ585 was directly proportional to the concentration of Hg2+ in the range 1–100 pM. The developed method had good linearity (y = 0.001x + 0.899; R2 = 0.968) under the optimized conditions with a limit of detection of 0.15 pM. The method was validated in real samples such as tap and lake water and could be used for the environmental sensing of Hg2+.

 Please wait while we load your content...
Please wait while we load your content...