Chiral separation and enantioselective degradation of trichlorfon enantiomers in mariculture pond water†
Abstract
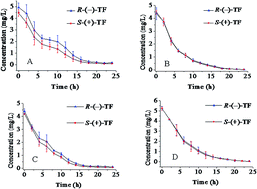
In this study, the dissipation and enantioselective degradation of trichlorfon (TF) enantiomers in mariculture pond water was investigated under various conditions using a chiral HPLC method. The results showed that the TF enantiomers dissipated following first-order kinetics in the mariculture pond water under natural, dark, sterile, and dark and sterile conditions. S-(+)-TF degraded more rapidly than R-(−)-TF under natural and dark conditions, whereas the dissipation of TF enantiomers in mariculture pond water was non-enantioselective under sterile, and dark and sterile conditions. Light and microbial activity played important roles in the dissipation of the TF enantiomers in mariculture pond water, and the TF enantiomers degraded faster at higher temperatures. Microbial activity was found to be responsible for the enantioselectivity under these conditions. The results of the study can be used to evaluate the environmental risk of TF at the enantiomer level.

 Please wait while we load your content...
Please wait while we load your content...