Peptide microarray-based fluorescence assay for simultaneously detecting matrix metalloproteinases†
Abstract
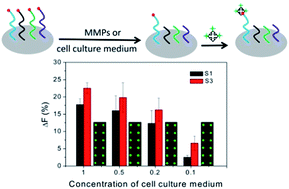
Matrix metalloproteinases (MMPs) are believed to play an important role in tumor invasion. Herein, a peptide microarray-based fluorescence assay has been proposed for simultaneously determining the activities of MMP-2 and MMP-9 through the strong binding affinity of fluorescein isothiocyanate modified avidin (avidin-FITC) with the immobilized biotinylated peptide substrate on the microarray. In the presence of MMPs, the biotin moiety is released from the microarray by enzymatic cleavage of the peptide substrate, resulting in a significant decrease of the fluorescence signal. Under the optimal experimental conditions, the fluorescence intensity changes (ΔF%) are proportional to the concentrations of MMP-2 and MMP-9 within the ranges of 50 pg mL−1 to 50 ng mL−1 and 50 pg mL−1 to 100 ng mL−1 in the enzyme mixture, respectively. The detection limits are 45 pg mL−1 for MMP-2, and 60 pg mL−1 for MMP-9. In particular, the activities of extracellular MMP-2 and MMP-9 are determined by the peptide microarray-based fluorescence assay, and satisfactory results are obtained.

 Please wait while we load your content...
Please wait while we load your content...