Quantitative investigation of the dynamic interaction of human serum albumin with procaine using a multi-way calibration method coupled with three-dimensional fluorescence spectroscopy
Abstract
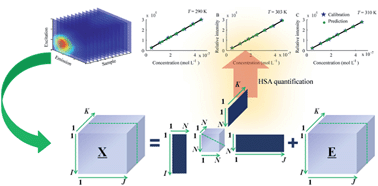
In fluorospectrophotometric studies on protein–drug interactions, the fluorescence intensity of proteins is often vulnerable to interference from ligands or newly produced complexes which exhibit significant fluorescence at the chosen excitation or emission wavelengths. Alternatively, this paper suggests an effective and sensitive method for quantitative determination of free human serum albumin (HSA) in a dynamic interaction system with procaine (PRO) and further investigation of their interaction mechanism using a multi-way calibration method coupled with three-dimensional fluorescence spectroscopy. A second-order calibration method realized the quantitative determination of free HSA in a dynamic system with overlapping spectra even in the presence of an uncalibrated interferent. The quantitative results were used to further calculate the binding parameters, including the binding constant, binding site number, thermodynamic parameters and nature of binding forces. Furthermore, the four-way excitation–emission–temperature–sample data were analyzed to investigate the effect of temperature on the interaction system studied.

 Please wait while we load your content...
Please wait while we load your content...