Development of a new clean methodology with ultrasound-assisted extraction for analysis of sodium in pet foods
Abstract
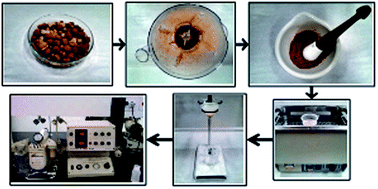
Sodium is essential to all living beings, including humans and animals; in higher heterotrophic organisms, it is responsible for regulating the osmotic pressure of tissues and maintaining the sodium–potassium pump. However, even though the presence of sodium is vital, in excess it can cause problems such as increased blood pressure and kidney stone formation. The official methodology for the analysis of sodium in pet foods employs corrosive acids and high temperatures during sample preparation, making the process time-consuming and prone to error. In this work, a new methodology is proposed for the extraction of sodium from pet foods, using ultrasonic irradiation to enhance the transfer of the analyte to solution, with subsequent determination by flame photometry. This new method, which is coherent with the principles of green chemistry, provided a linear range of 1–20 mg L−1 (R = 0.998), and limits of detection (LOD) and quantification (LOQ) of 0.26 and 0.90 mg L−1, respectively. Recoveries were in the range of 98.4–104%. The technique was successfully applied to different brands of commercial pet food and compared favorably with the official methodology (at a 95% confidence level). In comparative tests of the two extraction methods, the proposed methodology showed good repeatability, selectivity, precision, and accuracy.

 Please wait while we load your content...
Please wait while we load your content...