Facile, cost effective synthesis and DFT-based studies of substituted aryl hydrazones of β-diketones: a new selective fluorescent chemosensor for Co2+†
Abstract
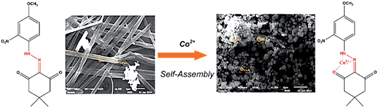
Intramolecular charge transfer (ICT) chromophores 2-(2-(4-methoxy-2-nitrophenyl)hydrazono)-5,5-dimethylcyclohexane-1,3-dione (CD1) and 2-(2-(4-methyl-2-nitrophenyl)hydrazono)-5,5-dimethylcyclohexane-1,3-dione (CD2) have been synthesized and used for the first time as chemosensors with reversible “on–off” sensing capabilities for biologically and environmentally significant Co2+, with detection limits of 3 μm to 7 μm. The new metal ion sensors that contain hydrazones of β-diketones have been synthesized and characterized by FT-IR, 1H and 13C NMR spectroscopy, scanning electron microscopy (SEM) and single crystal X-ray diffraction studies. The FT-IR and NMR spectral data clearly show effective intramolecular hydrogen bonding in all the synthesized substituted hydrazones. SEM was employed to investigate their morphology. A single crystal X-ray diffraction study confirms the exact structures of CD1 and CD2. A packing diagram explains the strong intramolecular hydrogen bonding in the CD1 and CD2 molecules. The absorption spectra are all similar, irrespective of the substituent and solvent. By comparison, the fluorescence is strongly dependent on the electronic character of the substituent. The sensors show excellent selectivity and sensitivity, with fluorescence enhancement in response to Co2+ over other cations in ethanol aqueous solution. Combined experimental and theoretical studies were conducted on their molecular structures using density functional methods (B3LYP) invoking the 6-31G basis set. The optimized geometric bond lengths and bond angles obtained by the DFT method show good agreement with the experimental values. The energies of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) have been predicted.

 Please wait while we load your content...
Please wait while we load your content...