Insitu thermal degradation of isopropanol under typical thermal desorption conditions for GC-MS analysis of volatile organic compounds
Abstract
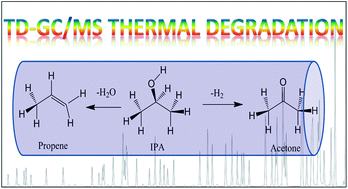
Thermal desorption (TD) coupled with GC has been widely used for the determination of airborne volatile organic compounds (VOCs) due to its effectiveness in detecting a wide range of compounds. However, although the residual compounds and artifacts of the most common thermal adsorbents used have been reported, the thermal decomposition of the VOCs themselves has not been well studied. We report here our observations on the possible thermal decomposition of isopropanol and its degradation products under the typical TD conditions used in the determination of VOCs by GC-MS. Acetone and propene were two products of such thermal decomposition observed when using the Carbopack B adsorbent, whereas only a small amount (0.05%) of these products was observed when using the Tenax TA adsorbent. Our results suggest it is possible that reported concentrations of isopropanol and acetone in indoor air may be affected by the thermal degradation of isopropanol if they are collected on adsorbents made of graphitized carbon black and determined using TD GC-MS. Caution therefore has to be exercised when selecting TD adsorbents in order to minimize the possible thermal conversion of VOCs.

 Please wait while we load your content...
Please wait while we load your content...